The Development of Clinical Document Standards for Semantic Interoperability in China
Article information
Abstract
Objectives
This study is aimed at developing a set of data groups (DGs) to be employed as reusable building blocks for the construction of the eight most common clinical documents used in China's general hospitals in order to achieve their structural and semantic standardization.
Methods
The Diagnostics knowledge framework, the related approaches taken from the Health Level Seven (HL7), the Integrating the Healthcare Enterprise (IHE), and the Healthcare Information Technology Standards Panel (HITSP) and 1,487 original clinical records were considered together to form the DG architecture and data sets. The internal structure, content, and semantics of each DG were then defined by mapping each DG data set to a corresponding Clinical Document Architecture data element and matching each DG data set to the metadata in the Chinese National Health Data Dictionary. By using the DGs as reusable building blocks, standardized structures and semantics regarding the clinical documents for semantic interoperability were able to be constructed.
Results
Altogether, 5 header DGs, 48 section DGs, and 17 entry DGs were developed. Several issues regarding the DGs, including their internal structure, identifiers, data set names, definitions, length and format, data types, and value sets, were further defined. Standardized structures and semantics regarding the eight clinical documents were structured by the DGs.
Conclusions
This approach of constructing clinical document standards using DGs is a feasible standard-driven solution useful in preparing documents possessing semantic interoperability among the disparate information systems in China. These standards need to be validated and refined through further study.
I. Introduction
In China, hospital information systems have been developed for more than 30 years and have gone through 4 application stages: single computer, department level, hospital-wide level and the current regional health information network level [1]. Because semantic interoperability was rarely considered in the development of the first three stages, the majority of encounter information, such as patient identifiers, demographic information, main patient problems, diagnoses, observations, medications, procedures, assessments, and expenditures could only be shared and exchanged within a specific hospital and could not be shared or exchanged between hospitals or external health institutions [2,3]. Therefore, developing standards for clinical information to promote semantic interoperability has become a priority in implementing the China National New Health Reform [4]. Thus far, 8 clinical documents, which are the most commonly used in China's general hospitals, have been medically identified with normalized contents and issued by the Ministry of Health (MOH) [5].
To be exchangeable, there must be standards for these documents to support semantic interoperability. Fortunately, several organizations have developed relevant standards, such as templates for the Continuity of Care Document (CCD) [6] in the Health Level Seven (HL7) [7], content modules [8,9] of Patient Care Coordination (PCC) in Integrating the Healthcare Enterprise (IHE) [10], and content modules of the Healthcare Information Technology Standards Panel (HITSP) [11], which were all based on the HL7 Clinical Document Architecture, Release Two (CDA R2) [12,13]. However, we cannot use them directly in our clinical documents because their development backgrounds and application conditions are different from those used in China. One difference is that the content of the templates and content modules do not suit our needs completely. Some information, such as medical expenses, administrative use, and quality assessment, are not present in the templates or content modules. Another difference is in the coding of value sets. Taking gender as an example, the codes in HL7 are "F = Female, M = Male, and UN = Undifferentiated", whereas the codes in China are "0 = unknown, 1 = male, 2 = female, and 9 = unaccounted." These codes have been widely used across China and have been a national standard (GB/T2261.1).
In this study, which is based on clinical record sheets in China's hospitals and references approaches from the HL7 CCD, the IHE PCC and the HITSP, we attempt to further develop a set of data groups (DGs) based on the CDA R2 as reusable building blocks to construct the 8 most common clinical documents in China's general hospitals. This will allow for structural and semantic standardization and promote interoperability.
II. Methods
1. The Contents of Clinical Documents
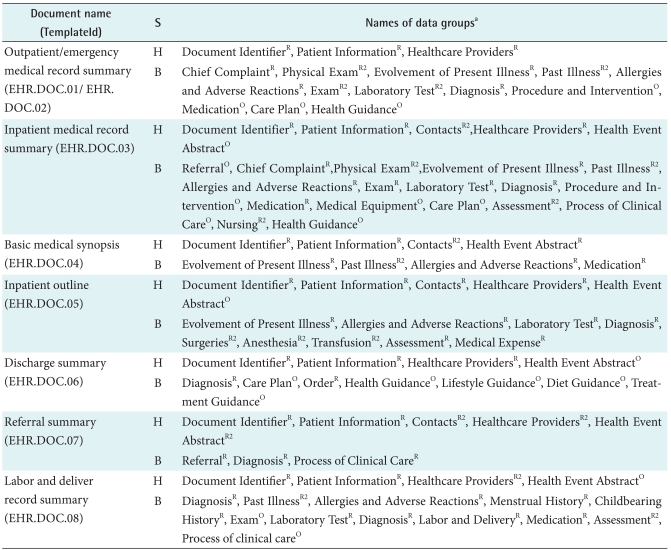
The 8 most common clinical documents in China's general hospitals are: 1) the outpatient medical record summary; 2) the emergency medical record summary; 3) the inpatient medical record summary; 4) the basic medical synopsis (a brief summary of medical activities concerning the evolvement of illness, including examining, diagnosing, and treating); 5) the inpatient outline (summary information during a hospital stay, which is usually as the first page of a paper-based medical record after discharge); 6) the discharge summary; 7) the referral summary; and 8) the labor and deliver record summary.
2. Chinese Health Data Dictionary
The Chinese National Health Data Dictionary (CNHDD) is a metadata repository that must comply with the standards for the construction of databases and health information systems. The metadata in the CNHDD was generalized and abstracted from various health information systems and legacy systems, with each metadata describing attributes of data identification, definitions, collection, usage guides, references and administration. At present, more than 1,500 metadata are available, and these metadata can be browsed by visiting the website described in [14]. In this research, we acquired standardized contents of each data item in the DGs by matching each data item with the metadata in the CNHDD.
3. Formulation Process of the DGs and Clinical Documents
1) Step 1: Development of the DGs' architecture and contents First, 1,487 original clinical record sheets from 14 representative general hospitals, including 4 hospitals with more than 2,000 beds, 6 hospitals with 1,000-2,000 beds and 4 hospitals with 500-1,000 beds across the country were collected. After merging the original sheets and removing redundant elements, 145 clinical record sheets were formed [15]. This dramatic reduction in the number of elements resulted from similar clinical procedures in most hospitals. Second, the framework of Diagnostics [16] knowledge and the approaches of the HL7 CCD, the IHE PCC and the HITSP for assembling templates and modules were considered together to propose the DGs' architecture. Third, the proposed DGs were used to construct 145 clinical record sheets as a pilot study to test their integrality. If the DGs could not completely build these sheets, the DGs were returned to a redefinition process. Lastly, data items within the DGs were identified by combining data items from the original sheets with the DG's architecture. Data items from the sheets were categorized and arranged in their related DGs, and data items having similar properties were abstracted. For example, data items B-mode ultrasonography examination ID, X-ray examination ID, CT examination ID and other examinations IDs were abstracted to two data items of examination type and examination ID. The B-mode ultrasonography, X-ray, CT, and other examinations became the codes in the value set for examination type after the abstraction.
2) Step 2: Definition of the structure, content and semantics of each DG
In this study, the HL7 CDA was chosen as our standard to represent the semantics of DGs and clinical documents for two reasons: 1) the HL7 CDA is a document markup standard that specifies the structure and semantics of a clinical document for the purpose of exchange [13], which suits our needs, and 2) the HL7 CDA has been chosen as the data exchange standard by MOH in the Technology Solution of Establishing Hospital Information Platform for Electronic Health Record (EHR) in China; thus, our standards should comply with the MOH standards [17].
By mapping each data item of a DG to the corresponding data element in the HL7 CDA, the structure of the DG was acquired. By matching each data items of the DG with the metadata in the CNHDD, a standardized description of the DG's items were obtained. Based on both above results, the DGs' semantics were defined. All the data items in the DGs have corresponding data elements in the CDA, and 90% of the data items were standardized directly by matching them with the CNHDD.
3) Step 3: Construction of each clinical document with the DGs If one or more data item in each clinical document was found in a DG, they were replaced by the DG. Thus, the contents of the clinical document were changed from being comprised of data items to being comprised of DGs, upon which the structure and semantics of the clinical document were finally produced.
During the formulation process, 4 discussion meetings were held to discuss the integrity and rationality of the developed DGs and the accuracy and significance of the clinical documents structured by the DGs. Altogether, 25 people participated in the consultations, including MOH leaders, health information experts, senior physicians, surgeons and software development engineers. The formulation did not proceed to the next step unless results of the current step were approved by 95% of those consulted.
The formulation process of the DGs and clinical documents are shown in Figure 1.
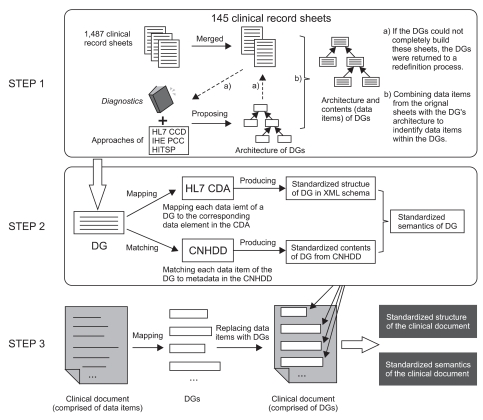
The formulation process of the data groups (DGs) and clinical documents, which includes three steps: development of the DGs' architecture and contents, definition of the structure, content and semantics of each DG, and construction of each clinical document with DGs. HL7: Health Level Seven, IHE: Integrating the Healthcare Enterprise, HITSP: Healthcare Information Technology Standards Panel, CNHDD: Chinese National Health Data Dictionary, CDA: Clinical Document Architecture.
III. Results
1. The Architecture and Contents of the DGs
Altogether, 5 header DGs and 65 body DGs, including 48 section DGs and 17 entry DGs, were proposed. The section DGs consisted of 17 section DGs and 31 sub-section DGs. Of the section DGs, Health Histories, Diagnosis, Procedure and Intervention, Medications, Assessment, Process of Clinical Care and Health Guidance all contained sub-section DGs (Figure 2).
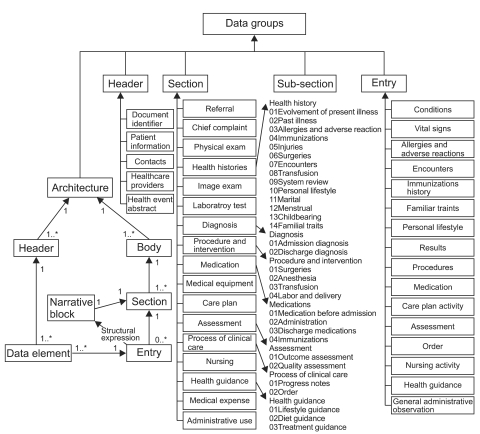
The architecture and contents of the data groups (DGs). Altogether, 5 header DGs, 48 section DGs and 17 entry DGs were proposed. Each DG contains one or more data items. A body consists of one or more section DGs. A section DG contains a single narrative block and zero or more entry DGs which represent the narrative block by structured data items.
Each DG conveys specific information. A header DG conveys identification information for documents, patients and involved providers. A body DG comprised of relevant section DGs conveys clinical report information. A section DG contains a single narrative block and possible (zero or more) entry DGs representing narrative content by structured data items (Figure 2). Thus far, narrative blocks of most of the section DGs can be represented by entry DGs, except for the Referral, Medical Equipment Use, System Review, Marital History, Menstrual History, Childbearing History and Progress Note narrative blocks. More entry DGs will be developed to represent these unstructured section DGs in future studies.
2. The Internal Structure, Content and Semantics of DGs
1) The standardized structure of DGs
The contents of 5 header DGs were structured by 12 data elements in the HL7 CDA. Data elements of typeId, templateId, id, code, title, effectiveTime, confidentialityCode and author were combined to represent the DG Document Identifier, recordTarget represents Patient Information, participant represents Contacts, documentation of represents Healthcare Providers and component of represents Health Event Abstract.
The contents of 65 body DGs were structured by the elements within component. Each section DG has one or more templateIds specifying its identifier, a code specifying the type of narrative block with Logical Observation Identifiers Names and Codes (LOINC) [18], a text that describes the content of a narrative block, and possible entry DGs representing the narrative block of structured data items. When matching to LOINC, most narrative blocks have matching LOINC codes, especially for those related to laboratory tests. For a few narrative blocks that have complex contents and cannot be matched completely with a LOINC code, we split them into several simple parts that have specific LOINC codes to be matched and use several components to represent them accordingly.
Meanwhile, the data items of 17 entry DGs are represented by data elements of the CDA classes act, encounter, observation, organizer, procedure, substanceAdministration and supply. The 16 former entry DGs are used to describe information related to clinical activities, while the last entry DG, General Administrative Observation, is developed exclusively to describe information for hospital management, such as the length of hospital stay, the cure rate, and the death rate.
2) Standardized contents of the DGs from the CNHDD
Standardized metadata attributes of the data items in the DGs were acquired after matching each data item of a DG with corresponding data elements in the CNHDD. The matched data items are instances or specializations of the data elements in the CNHDD. For example, the data item doctor's name is an instance of the data element name, and the date of allergy is an instance of date. During the matching process, 95% of the data items have direct corresponding matches in the CNHDD, and 5% of the data items cannot be matched or are only mapped to codes in the value sets. Regarding these problems, the data items in the DGs are returned to the redefinition process, or the data elements and value sets in the CNHDD are added or adjusted after discussions with experts in developing and maintaining the CNHDD.
3) Standardized semantics of the DGs
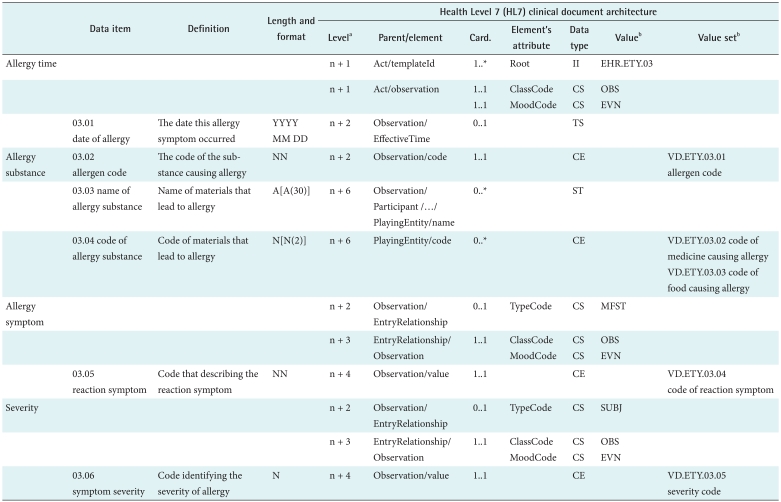
Based on standardized structure and content, the semantics of each DG were acquired. For example, Table 1 shows the matched standardized metadata attributes of the entry DG Allergies and Adverse Reactions and its representation structured by the HL7 CDA. The values of attributes (including definition, length and format, data type and value set) for the data items are derived from the CNHDD. In line with the CNHDD, the contents of Parent/element, card. (cardinality), element's attribute and value are defined and represented by the HL7 class of act and nested observation. Meanwhile, the relationships of allergy substance, symptom and severity are connected by the element entryRelationship, and their relationships are specified by MFST and SUBJ.
Length and format is described in the same manner as the descriptions used in METeOR [19]; data type is the HL7 Version 3 data type [6], and value set is the code collection for data item whose data type is CE. In addition, the value sets were standardized by referring to the ISO/IEC 11179-3 [20]. Their coded values are defined according to the sequence of a national standard (e.g., sex code from GB/T2261.1-2003), several code systems (e.g., diagnosis code from ICD-10), 8 clinical documents, collected clinical record sheets and the CNHDD.
Using instance data, an XML file of the DG can be produced. Figure 3 shows the XML file of the entry DG Allergies and Adverse Reactions with actual data (allergy substance-penicillin, allergy symptom-hives).
3. Semantics of the Clinical Documents Structured by DGs
One or more data item in each clinical document can be mapped to corresponding data items in the DG and then replaced with that DG. For example, the data item provider's hospital name and provider's department name were replaced with Healthcare Providers (EHR.HRD.04). Type of laboratory test, name of laboratory test, value of laboratory test and measurement unit were replaced with the section DG Laboratory Test (EHR.SEC.06). Finally 8 clinical documents were all structured by a number of DGs (Table 2), based on which standardized structures and semantics of the documents for semantic interoperability were produced in the HL7 XML format. For example, Figure 4 shows the detailed structure and semantics of outpatient medical record summary document structured by 3 header DGs and 12 section DGs in XML Schema.
IV. Discussion
1. Localization of the HL7 Standards in Our Research
The methodology of building shareable clinical documents using the HL7 CDA is a recognized solution [21,22], yet we do not use it completely because the attributes of the data elements and codes of the value sets in the HL7 do not completely suit our needs. Therefore, we customized the HL7 CDA in two ways in our research. According to our business needs and on the condition that the architecture of the HL7 CDA remains unchanged, one way to customize the HL7 CDA was to adjust the attributes (e.g., cardinality, data type) of data items in the DGs, and the other method was to redefine codes of parts of value sets. Therefore, a contribution of our research is to promote the use of the HL7 standards in China.
2. Characteristics of DG-based Clinical Documents
Based on DGs, clinical documents have certain characteristics. First, clinical documents constructed by DGs will be structured, enabling embedded information to be more complete and accurate [23,24]. Second, more than just these eight clinical documents can be built by flexibly reusing the DGs. The architecture of the DGs can stay stable merely by adding codes to value sets and adjusting the data items' attributes in the DGs when more documents need to be built. Third, DGs and data items that are irrelevant to clinical documents will be excluded by defining their attributes of optionality and cardinality, which can keep clinical documents clear and concise.
3. Differences between DGs and the Components of CCD, PCC and HITSP
Almost all the sections of the CCD, the PCC and the HITSP can be matched to corresponding section DGs except for two: the section describing medical care expenses and the section representing hospital management information. The CCD, the PCC and the HITSP use Payers section to specify organizations or individuals who may pay for a patient's healthcare, while we use Medical Expense section to describe actual expenditures that have been paid. We use Administrative Use section to describe the information used for hospital management (e.g., cure rate, death rate), whereas this section is absent in the sections of the CCD, the PCC, and the HITSP. Furthermore, entries also differ as a result of the differing sections. In conclusion, these differences come from business variations among different cultural and language backgrounds.
Acknowledgements
This work was supported by the Research Grant (Grant No. 81102202; 81171427) from National Natural Science Foundation of China, by the Research Grant (Grant No. 2009JM4028) from Science Foundation of Shaanxi Province, and by the National Science and Technology Infrastructure Program from the Ministry of Science and Technology of China (Grant No. 2008BAI52B01).
Notes
No potential conflict of interest relevant to this article was reported.