|
 |
- Search
| Healthc Inform Res > Volume 17(4); 2011 > Article |
Abstract
Objectives
This report describes the development process of a drug dosing database for ethical drugs approved by the Korea Food & Drug Administration (KFDA). The goal of this study was to develop a computerized system that supports physicians' prescribing decisions, particularly in regards to medication dosing.
Methods
The advisory committee, comprised of doctors, pharmacists, and nurses from the Seoul National University Bundang Hospital, pharmacists familiar with drug databases, KFDA officials, and software developers from the BIT Computer Co. Ltd. analyzed approved KFDA drug dosing information, defined the fields and properties of the information structure, and designed a management program used to enter dosing information. The management program was developed using a web based system that allows multiple researchers to input drug dosing information in an organized manner. The whole process was improved by adding additional input fields and eliminating the unnecessary existing fields used when the dosing information was entered, resulting in an improved field structure.
Since the first implementation of electronic medical record systems to real world applications, it has been suggested that a digitalized drug utilization review (DUR) at the time of drug prescription or dispensing would be an effective way to reduce errors in medication utilization [1-4].
In the establishment of a DUR system, the most difficult step is digitalizing the text based information on the dosage range check module (DRCM) for each indication. It is also very important that the completed database is based on sources that professionals in the field (including physicians and pharmacists) are able to trust. While several international and domestic institutions have developed their own dosage databases and employed them in daily practices, there are considerable variations between the dosage guidelines that they provide. Of course, there are a number of dosage databases that have roots in a national standard based on government official resources, such as market approval information. Although most of these information sources (e.g., MICROMEDEX) are internationally available, access to their content is inevitably limited to a considerable degree. Hence, there has been an urgent demand for a national database that can provide trustworthy drug dose information to professionals.
In this work, therefore, the authors aim to develop a DRCM on which a dosage database for locally marketed ethical pharmaceuticals can be built based on the approved information on indications and predefined age category dosages. By doing so, the foundations for establishing a standardized DUR system at a national level can be developed, and better, safer, drug therapy will be available to the general public.
The system used in this study is unique in terms that it provides the dosage information for each indication defined in the original Korea Food & Drug Administration (KFDA) label. The dosage information in the labels were separated into three parts; dosage value (e.g., 30), unit (e.g., mg), and a number tag for each indication to facilitate the direct comparison with prescriptions written by doctors. Furthermore, instead of managing this data in generic commercial software (e.g., Microsoft EXCEL), a standalone database was developed for the efficient handling of the data in terms of input, management, and regeneration of information. In particular, the database was designed to decipher the subtle differences in data input that may occur for different people and standardize them (e.g., automatically reading the input 100MG as 100 mg; understanding 100IU as 100 I.U.). Without this function, when large amounts of data are accumulated, reprocessing and/or re-purification of the information would be inevitable. Therefore, the system presented in this study is likely to eliminate/minimize problems associated with the inefficiency.
An advisory committee comprised of doctors, pharmacists and nurses from Seoul National University Bundang Hospital was formed to oversee and advise the construction of the DUR database. The committee also included pharmacists familiar with drug database construction/analysis/and planning, the KFDA officials that are in charge of pharmacy affairs, and software developers from BIT Computer Co. Ltd. (i.e., one for Javascript and another for database construction). Monthly meetings were regularly held for communication, discussion and consultation on the purpose and contents/scope of the software.
Currently, the nomenclature and unit information are not fully standardized in KFDA labels. Therefore, the dosage/usage information in these labels had to be processed and analyzed before writing the data into the database. This procedure decided what items would be included in the database and what information considering that item would be introduced or left out, allowing the new database to maintain a consistent structure. Whenever inconsistencies were found in the labels, especially in usage or dosage, an advisory committee meeting was called for discussion and consultation.
To provide a standard working environment, the following process standards were implemented to enhance the reliability of the database while securing the convenience of workers.
Usage/dosage information was conceptually structured and processed so that information could be easily separated into discrete parts. The advantage here is that inconsistencies in dosage information can be quickly identified by the manager.
Since typographical errors may affect the quality of the database if information is processed in a raw text format, the separation of data into number and unit fields is necessary. In particular, subtle differences that may occur when inputting units, which are mainly created by the diversity of the people using the program, may also affect the quality of the database. Therefore, only pre-registered units were allowed to be used in the processing of units.
During database construction, each drug item had to have a complete field of information filled out. This means that for every item, information on its contents (e.g., major ingredients in the drug products), available forms of dosage (e.g., powder, solution and tablet), and precise dosages in appropriate units for each form of dosage (e.g., mg or mL) was processed, allowing every possible dosage unit in the prescription to be inter-convertible in a timely fashion.
For certain drugs, the drug product contains a given salt form of the drug. For example, 12.79 mg of amlodipine mesylate monohydrate would make a 10 mg tablet of pure amlodipine. For the database introduced in this study, a field for total dosage (i.e., salt-included content) was provided for the easy conversion of the actual dosage of the drug (i.e., free acid or base).
If possible, the dosage information of combination drugs was further processed for easy conversion and flexible use. For example, conventional Sinotrim syrup contains 8 mg/mL of trimethoprim and 40 mg/mL of sulfamethoxazole (i.e., unprocessed data). However, the dosage for the product was converted to 2.5 mL of Sinotrim syrup per kg body weight, considering the fact that the dosages for trimethoprim and sulfamethoxazole were 20 mg/kg and 100 mg/kg, respectively.
Since some drugs, such as parenterals and ophthalmic drops, have a solution form (or dissolved immediately before use), the total volume of injection (or application) determines the overall concentration of the formulation. Therefore, detailed information on the volume of administration was provided in the database used in this study.
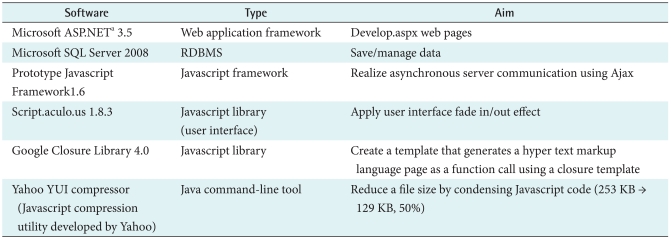
Since information is processed by pharmacists throughout the hospital, it is very convenient and time saving if their inputs are gathered in a web environment. In addition, under these circumstances, managers in the project can oversee progress and statistically analyze the data from remote locations. This type of work environment enabled pharmacists to accomplish their objectives effectively in a punctual manner. Furthermore, this systematic and integrated web environment let managers centrally process data much more easily than generic commercial software.
1) Using AJAX (asynchronous Javascript and XML; e.g., Google), the pharmacists were provided a convenient input environment, the managers an efficient review environment, and the operators a comprehensive managerial environment [5].
Since there are a number of similar generic products for a given drug, the usage/dosage would be identical for each of these cases. Therefore, without the need to reprocess identical information, previously processed data can be easily retrieved and appropriately reused to reduce processing time.
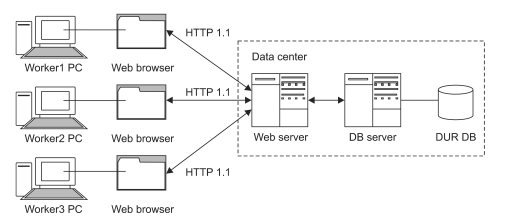
The web based DUR data management system was designed to have a client-server architecture. This enables pharmacists to process and manage drug information on a PC running a generic web browser (i.e., client). All processed data is then stored in the DUR database (DB) of the DB server (Figure 1), meaning a web server processes the request for data. The web browser running on the PC of the client communicates with the web server under a HTTP 1.1 protocol, and in this regime, the requests for data and responses to this requests are exchanged in a manner similar to any web page access. In the construction of the DB used in this study, only one web server and one DB server was used instead of multiple web and DB servers since the system only used intermittent data processed at irregular intervals. Because of this small workload, there was no need to set-up multiple servers (e.g., load balancing, master-slave data replication), reducing the complexity of design and allowing its construction in a short period of time.
Since KFDA labels frequently contain unstructured natural language texts, pharmacists (i.e., inputters) are likely to analyze the information on the tags and then convert/process them into the structured data/database. Under this situation, the addition of an extra field may become necessary. The system was made to be sufficiently flexible when adding these extra fields. The primary objective was to convert the data contained in the articles of the label and properly store them in the database by creating a DUR entry (Figure 2).
In general, the usage and dosage information of drugs approved by the KFDA, except for those excluded by the criteria given below, were subjected to data entry. Since certain articles in the label may differ slightly even under identical ingredient codes, all data was processed based on the Drug Item Base Code.
Exclusion criteria are combination drugs containing three or more active ingredients in one product, drug products for export, withdrawn drugs, radioactive drugs, X-ray contrast media, diagnostic testing agents, dialysis irrigating solutions, vaccines, blood-derived drugs, biological drugs, herbal medicines, and orphan drugs (biological drugs, herbal medicines, blood-derived drugs, radioactive drugs, diagnostic testing agents, subcutaneous injections, and intramuscular injections).
Ezdrug (http://ezdrug.kfda.go.kr), an official drug information database from KFDA, was used to process dosage information. Since it took approximately two minutes to display the information page (e.g., indication, usage/dosage and warning/precaution) of a particular drug product, the cumulative delay in the process of information collection was significant. Therefore, relevant information was collected by analyzing the HTML source code of the Ezdrug webpage using Internet Explorer's view source code menu. By entering CCBAA02L2 in the variable 'CMD' of http://ezdrug.kfda.go.kr/kfda2 and the Drug Item Base Code in the variable 'itemseq', a specific page in Ezdrug can be directly accessed. For example, the url 'http://ezdrug.kfda.go.kr/kfda2?cmd=CCBAA02L2&itemSeq=199300694' gives direct access to the 'Albis Tab.' drug information page. Making some optimizations, this access time can be reduced to approximately 30 seconds.
This study finally built a database containing the dosing information for the 4,454 ethical injections and 12,450 ethical oral preparations currently being sold in the Korean market (July 2009). This accounts for 16,994 items in total, excluding the drugs omitted by the criteria mentioned above (e.g., radioactive drugs, X-ray contrast medium).
Manager mode provides a module to check the propriety of prescribed dosages in specific cases. The criteria for this type of validation are established by building databases grouped by age and indication. Market approved information (dosage, dosing guidelines and clinical applications) was employed as the default standard of for dosages. The basic guidelines for manager mode and its key features are as follows:
In order to build a body of information for various types of dosage, a term 'In case of' was created as an information unit. The concept of this term is as follows; 'in case of ~, the dosage schedule is ~'. As a detailed 'In case of' report tends to make dosing (numerical data) information more precise, a single dosing informa`tion should be correlated to a single specific 'In case of' report if possible, because this could reduce variations across operators caused by individual misunderstandings.
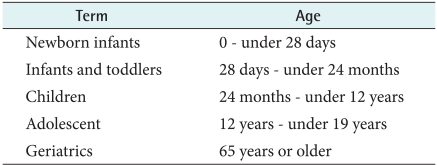
While age standardization is important, it is difficult to define a standard age category in current drug market approved information. Several terms such as neonates, infants, toddlers and children are mentioned, which have no numerically specification. Also, there are ambiguous terms regarding the aged population that tend to have little consistency. To reduce the confusion from these ambiguous age terms, this study employed the age group guideline set by the KFDA in November 2008, which is based on the ICH guideline (Table 2).
Information on the patients' weight and BSA is important in determining their dosing schedule. Thus, the system was developed to require the input of such information.
(1) Single dose or one day dose: The maintenance dose is entered when there is information on both the initial dose and the maintenance dose. Only the initial dose is entered when there is no information concerning the maintenance dose. Also, the concept of an allowable maximum dose was implemented, along with the usual minimum and maximum dose.
(2) Dose and dosing interval within a single course of therapy: The concept of 'a single course of therapy' was introduced to indicate the case that requires several repeated inputs of a particular medicine in a relatively short time. For instance, if a medication is given as an IV injection at doses of 1 mg/kg every 10 minutes until the symptoms resolve, the maximum interval and the minimum interval within a single course of treatment are both equivalent to 10 minutes. This field was newly defined in the present study because it could be an important concept that could check whether emergency injections are appropriate in terms of dosing.
(1) Duration of therapy: The 'duration of therapy' was used to indicate the relatively long treatment periods mentioned in the standard period the KFDA market approved information recommends to achieve a therapeutic purpose. For example, if it suggests 4 weeks of treatment of Furing® tab (Phendimetrazine tartrate 35 mg), the duration of therapy is 4 weeks.
(2) Infusion time: Infusion time denotes to the time taken for infusing a single dose of medicine. For instance, infusion time for an injected drug is 30 minutes if each dose is infused for 30 minutes. This field can be used to check infusion speeds.
(3) Dosing intervals: The dosing interval denotes the time between two doses in cases where this time is significantly longer than the time of a single course of therapy described above. The dosing interval is 8 hours if 100 mg of a certain medication is treated every 8 hours.
A drug holiday is the period in which a patient stops taking a medication(s) for a period of time because of drug accumulation or other adverse effects. Because it is a different concept from the dosing interval mentioned above, new fields were created so operating staffs are able to input information on the minimum and maximum duration of treatment, drug holidays, and the number of treatment cycles.
Manager mode also allows medical personnel to check if dosing adjustment is required due to renal or hepatic dysfunction.
It may be necessary to add new field to input strange DUR information into database. In that case, Manager mode can be updated to add new field into database with slight editing in source-code and database.
(1) KFDA: The KFDA information can be acquired by either connecting to a link on the web or by directly referring to texts in the database.
(2) References: There are tools that allow users to look up further dosing information in the AHFS Drug Information, Pharmaceuticals and Medical Devices Agency and Martindale.
(3) Notes: There was a field for notes where operators left their opinions, abnormal issues, etc., making references for reviewers.
Log in to the DUR data managing web (http://www.druginfo.co.kr/dur/admin) to access the system.
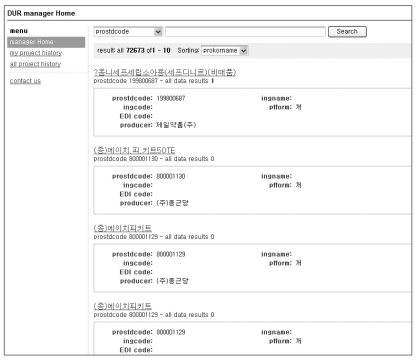
(1) To search any product at the manager home after connecting, select a searching option. The default is the national product code (Figure 3).
(2) The list of search results appears as shown in Figure 4. The total number of search results is presented at the top of the page. The maximum number of products that can be presented on a single page is 10. By clicking a product name, operators are transferred to a page where they can add and manage data.
(1) Data Input: Choosing the link [Data Input] in the left menu bar opens a screen for a data entry mode (Figure 5). Click the [Save] at the top to save data after finishing data input.
(2) Managing data: The new system provides tools for operators to review, amend, and remove data from the database.
(3) Data import: The tool [Data Import] is a function that allows operators to copy information from previous data to the current entry form without entering any data [Data input]. This is useful to enhance work speed when the dosing information of one product is identical to a data set already entered. Clicking the [Data Import] button after entering the national product code for the previous data set that the operators want to copy will load the copied data to the current entry form.
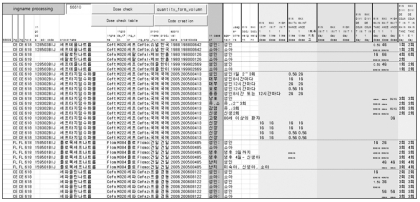
(4) Data browsing: Data and data entry times can be checked and searched through the [Work Breakdown] function in several ways. Clicking the link [All Work Breakdown] in the left menu presents the total number recent operations and lists them in chronological order. To perform a detailed browse in a different way, operators can choose various searching options such as [worker] to search for progress made by that specific worker.
1) Dosing information in the established database can be exported into an Excel spreadsheet for printing. Figure 6 presents an example of the exported data showing drug profiles, 'In case of' information, and original texts.
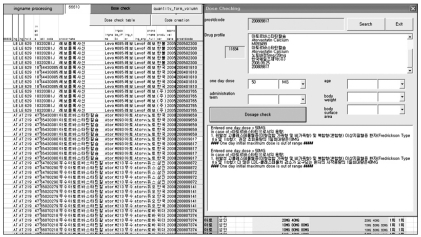
One important purpose of this project was to set up a standard for a dosage check and, moreover, to utilize this established standard in the DUR program. To give a trial performance in a simple manner, a new dosage checking program was developed based on the established dosing information. In other words, a Visual Basic for Application (VBA) program that checks the propriety of the dosages was loaded onto a converter program. As shown in Figure 7, users can open a window for a dose check easily by clicking the [Quick Dosage Check] button in an output sheet. When a window is popped up, users can retrieve a drug profile by entering the national product code of the drug they would like to check (Figure 7). Then, users can input a dose (that users want to check) in a pertinent option(s). Clicking the [Check] button shows the results in a separate textbox.
As prescriptions reimbursed by public funds are rapidly increasing in the Korea National Health Insurance, constant efforts to utilize information technology have been made as an attempt to improve documentation accuracy as well as to save time. However, computerized DUR systems have never been developed at a nationally level, and these systems were always developed by local organizations. This is thought to come from the following potential factors. First of all, every hospital has its own operating system. As a result, standard systems that can be applied to all hospitals are hard to develop. Also, professionals in the field tend to underestimate the effectiveness of digitized DUR systems due to the lack of information provided to them [8].
Therefore, this study built a dosage database for locally marketed ethical pharmaceuticals based on KFDA approved information on indications and predefined age category dosages.
The system was developed in three steps. The first step was to build a web-based manager mode in which multiple operators were able to connect at the same time to build a dosing database. The manager mode program had to be easy for users to learn and capable of doing the users needs while also being secured and automatically backed up. The next step was to establish a real dosing database. This stage was challenging and often required changes in the pre-established manager mode program. The last step was to develop a tool that could review the established data and to develop a method to submit the database as an outcome of the study. Lessons learned from this study are summarized in the following:
Redundancy denotes cases where there are more than two ways to express the dosage. Examples may include similar and identical expressions to those shown in Figure 8. In such cases, this study tried to stick to the official market approved expression as much as possible. In the future, it is recommended that an independent and consistent converting module should be built in the DUR program.
While a field that shows dosage dynamics (the trend of dosage change over time) is important for DUR services, the present study failed to reflect it due to limitations in time.
Beyond the simple implications of increase and/or decrease, the information included in the new field should contain several different aspects of dosage change such as time, quantity, and frequency. Hence, the technique in demand may be to first produce database templates for every field in the basic information database and then assign templates to each newly created dynamic dosage category when new categories are needed.
Applying the new concept of 'In case of' to dosing information was challenging. To keep decision making in this line of thinking objective, loading a standardized guideline onto the manager mode program seems necessary in future research.
Digital codes from Systematized Nomenclature of Medicine (SNOMED) or ATC codes may benefit the future system in terms of efficiency in comparison with the text information employed in this study. To reduce the confusion caused by the frequent adding or deleting of dosage fields that often delayed the working process, it is recommendable to consider an independent MARKUP language that digitalizes dosing information more easily as shown below:
250 mg, once per day, maximum 500 mg ŌåÆ day length=1 times=1 norm_dose=250 dose unit=mg max dose=500 dose unit=mg
Acknowledgements
This research was supported by a grant (09142KFDA234) from Korea Food & Drug Administration in 2009.
References
1. Rich D, Menke J, Fisher D. Dose range checking in a computer order entry system. AMIA Annu Symp Proc 2003;985.

2. Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, Classen DC, Bates DW. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 2007;14:29-40. PMID: 17068355.

3. The U.S. Pharmacopeia Drug Utilization Review Advisory Panel. Drug utilization review: mechanisms to improve its effectiveness and broaden its scope. J Am Pharm Assoc (Wash) 2000;40:538-545. PMID: 10932464.

4. Teich JM, Merchia PR, Schmiz JL, Kuperman GJ, Spurr CD, Bates DW. Effects of computerized physician order entry on prescribing practices. Arch Intern Med 2000;160:2741-2747. PMID: 11025783.

5. White A. Measuring the benefits of Ajax [Internet]. Inter.com c2011;cited at 2011 Apr 1. Available from: http://www.developer.com/xml/article.php/3554271

6. Gravano L, Ipeirotis PG, Koudas N, Srivastava D. Text joins in an RDBMS for web data integration. WWW '03 Proceedings of the 12th International Conference on World Wide Web 2003;New York, NY: Association for Computing Machinery; p. 90-101.

Figure┬Ā1
A client-server architecture in a drug utilization review (DUR) data manage system. DUR data manage system is constructed by connecting a database (DB) server to web server in which a manager program is built, for the purpose of facilitating multiple user's inputting and centralized managing DUR data. This client-server system enables multiple users input and manage data effectively rather than stand alone systems or files.
Figure┬Ā2
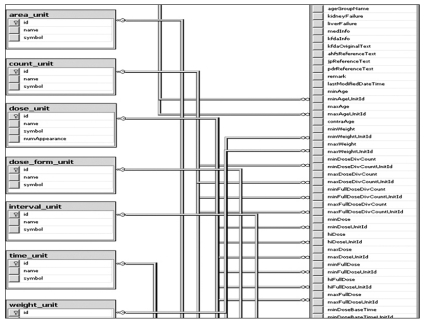
Diagram of the relationship among dosing information tables. A dosing information table is a kind of database (DB) blueprint by which workers decide what information should be extracted from usage and dosage and then how it is processed. We made an effort to make the DB useful for dosing check-up substantially without distorting any meaning of original usage and dosage in a DB making process. Further continuous improvement in DB structure is needed.
Figure┬Ā3
Drug utilization review (DUR) manager home 1. DUR manager home supports diverse options search for DUR data. It also enables operators to manage "my work list" and "whole workers' work list".
Figure┬Ā4
Drug utilization review manager home 2. It shows the searched sum-up information on work-list data and searched results. And it also displays a current inputting work status on DUR data. Product's name and other list information are written in Korean becasue our Database was targetted to drugs saled in Kore.

Figure┬Ā5
Drug utilization review manager home 3. This picture shows the "input mode on a case basis" for entering detailed information on a specific drug. It supports a quick move to diverse input categories. Separating numerical values and unit values in the beginning of data input made workers possible to enter the same unit values consistently and check up the dosing data instantly.

Figure┬Ā6
An Excel spreadsheet of dosing information from the established database. Inputted data can be printed in an Excel sheet form. And it also can be converted in any forms (easy to understand and handy to manage) by analyzing an enormous amount of inputted information via Visual Basic for Application program developed to process a data. DUR contents are written in Korean, because we analyzed contents based on Korean Food and Drug Administration's drug label infomation.
Figure┬Ā7
An Visual Basic for Application (VBA) example of Quick Dosage Check in an exported drug utilization review (DUR) data. VBA code can enhance utilization of DUR data exported in the excel sheet form. It can process data, and also check dosage data in an excel environment. VBA code makes specific data possible to be converted into a variety of media (HTML, MDB, TXT, JAVASCRIPT, etc.) and to be modified into a lot of forms. VBA code helps managers to makes the better use of DUR data.
Figure┬Ā8
Examples of redundancy. One can find many occasions in which the same or similar expressions (in the label) slightly differ in numerical values or unit values when inputting dosage information on a case basis. This situation is defined as redundancy of drug utilization review (DUR) information. In any circumstances, the same results should be come out by processing regardless of the kind of values. This manager program has a unit conversion function by which unit information is separated and inputted independently.

- TOOLS
-
METRICS

-
- 1 Crossref
- 3,636 View
- 35 Download
- Related articles in Healthc Inform Res
-
Development of an Automatic Pill Image Data Generation System2023 January;29(1)
Research Development Using REDCap Software2021 October;27(4)
Development of Korean Rare Disease Knowledge Base2012 December;18(4)
Development of Renal Dosing System at a University Hospital2008 December;14(4)
Development of Korean Oriental Nursing Terminology Set2007 June;13(2)