Informatics as Tool for Quality Improvement: Rapid Implementation of Guidance for the Management of Chronic Kidney Disease in England as an Exemplar
Article information
Abstract
Objectives
Chronic kidney disease (CKD) is an important cause of excess cardiovascular mortality and morbidity; as well as being associated with progression to end stage renal disease. This condition was largely unheard of in English primary care prior to the introduction of pay-for-performance targets for management in 2006. A realist review of how informatics has been a mechanism for national implementation of guidance for the improved management of CKD.
Methods
Realist review of context, the English National Health Service with a drive to implement explicit national quality standards; mechanism, the informatics infrastructure and its alignment with policy objectives; and outcomes are describe at the micro-data and messaging, meso-patient care and quality improvement initiatives, and marco-national policy levels.
Results
At the micro-level computerised medical records can be used to reliably identify people with CKD; though differences in creatinine assays, fluctuation in renal function, and errors in diabetes coding were less well understood. At the meso-level more aggressive management of blood pressure (BP) in individual patients appears to slow or reverse decline in renal function; technology can support case finding and quality improvement at the general practice level. At the macro-level informaticians can help ensure that leverage from informatics is incorporated in policy, and ecological investigations inform if there is any association with improved health outcomes.
Conclusions
In the right policy context informatics appears to be an enabler of rapid quality improvement. However, a causal relationship or generalisability of these findings has not been demonstrated.
I. Introduction
1. Chronic Kidney Disease
Chronic kidney disease (CKD) is a common and generally symptomless condition affecting 5%-10% of the population. CKD is important because this condition is associated with an increased risk of cardiovascular morbidity and mortality [1], hospitalisation [2], and progression to end-stage renal disease. CKD like many long term illnesses is more common in older age-groups. It is more common in females but the proportion of males increases as renal function declines [3,4]; with males more likely to develop proteinuria [5]. CKD differs across ethnic groups, and with increased deprivation. CKD is associated with heart disease, heart failure, hypertension and diabetes. Strict control of systolic blood pressure (SBP) is known to slow progression [6,7] and may be cost effective [8,9].
2. IT in the English National Health Service
The English National Health Service (NHS) is highly computerised. The English National Programme for IT (NPfIT) was highly ambitious and expensive, succeeding in some areas but failing in others [10]. However, its legacy has been a national unique ID used throughout the health system (NHS number), a minimum dataset is collected nationally for each hospital episode, and nearly all encounters with primary care are recorded on computer at the point of care [11]. Primary care has a registration based system, meaning that patients can only register with one practice. Practices are computerised and electronic patient record (EPR) systems are used almost universally at the point of care [12]. Repeat prescribing data are complete and electronic links to pathology labs means that test results are sent directly into practice EPR systems. The UK primary care pay-for-performance (P4P) scheme rewards quality based upon routinely collected data measures; this in turn has further improved data quality [13]. The provision of a common data extraction platform for the different brands of EPR systems (Morbidity Information Query and Export Syntax, MIQUEST) make it possible to run a common data extraction query across different practices; and whilst there are inevitable problems with the extraction process [14], it is possible to reliably combine and process routine data [15,16].
I became involved in CKD research because renal specialists were interested in finding people with CKD from routine primary care data lacked the technical expertise to do this. This started a journey to which I, and my research colleagues, contributed informatics and primary care expertise. This review article is a realist review of how informatics has been a mechanism for national implementation of guidance for the improved management of CKD.
II. Methods
1. Overview, a Realist Review
This review was conducted as a realist review; developing explanatory analyses of why and how an informatics mechanism-in reality a complex intervention maximising use of available IT-might have succeeded or failed in the context of improving the management of CKD the English NHS.
The realists' mantra is "Context (C)" plus causal link with an appropriate "Mechanism (M)" results in an "Outcome (O)" [17]. This can be represented as a formula: C + M = O. Part of the realist perspective is that effects are reported according to the three Ws: "What works, for Whom, and in What circumstances." For the purpose of this analysis we considered:
Context to be the English NHS, a state funded national health system. It is free at the point of delivery and aspired to deliver and evidence-based service based on explicit national quality standards.
Mechanism included health IT and informaticians.
Outcomes were explored using Donabedian's classic evaluative framework: looking at structures, processes and any change in disease outcomes [18].
2. Exploring Mechanisms and Outcomes at Micro-, Meso-, and Macro-levels
The mechanisms and their related outcomes are described at the micro-, meso-, and macro-levels. We used a classification developed as part of a European project to assess readiness to participate in research [19,20].
1) Micro-level
The micro- or data-level data items are those which need to be semantically interoperable within this context. Critically important in this review aredata defining a diagnosis of CKD and including measures of renal function. This will include codes for CKD diagnosis; estimated glomerular filtration rate (eGFR)-a measure of renal function used to diagnose CKD [21]; key comorbidities including hypertension and diabetes. The recorded incidence and prevalence of the disease will be defined from the recording of diagnostic codes or the number of people with a reduced eGFR (stages 3 to 5 CKD are defined by an eGFR <60 mL/min).
2) Meso-level
The meso-level is the local or practice delivered care level, and includes the medical record and impact of the method of data extraction. Data can be extracted for research and locality audits using MIQUEST, a Department of Health data extraction tool. A different method is used to count cases for the P4P indicators. This is done using an audit tool which counts cases flagged with specific codes. It then uploads a count of the number of eligible people on the disease register and for their quality of care. The P4P tool therefore provides a measure of quality without passing on any personal data.
3) Macro-level
The macro-level is the health system, social and cultural context constraints within which careis provided. In the English NHS there is explicit national guidance. This takes the form of guidelines produced by the National Institute for Health and Clinical Excellence (NICE); for certain key chronic conditions there are National Service Frameworks and national clinical leads "Tsars." P4P for chronic disease management is also in plae. There are increasing levels of regulatory compliance with physicians and other health professionals being appraised and needing to revalidate to continue their professional practice. Notwithstanding this guidance and increasing regulation clinicians in England remain relatively independent, with most general practitioners self-employed contractors.
3. Data Sources and Study Periods
The primary data source used in this study was the Quality Improvement in Chronic Kidney Disease (QICKD) trial data. These data were collected between 2008 and 2011 from a national representative sample of just over 1 million patients in primary care, of whom around 7% had CKD [4,22]. We had also conducted an associated systematic review [6].
Prior to this were involved in the New Opportunities for Early Renal Intervention by Computerised Assessment (NEOERICA) study, which demonstrated that it were possible to identify people with CKD from primary care computer records, this was carried out 1998 to 2003; using records from an adult population of 130,226 adults [8,23,24].
Where we conducted studies on a National Basis, we used nationally publically available datasets from the UK Renal Registry (www.renalreg.com) and the NHS Information Centre (www.ic.nhs.uk). The "macro (national) data" reported for our ecological study we used between 2003 and 2008 [25].
The single patient case study, of the impact of actively managing BP in CKD, was taken from the authors practice, using data taken between 2006 and 2012.
III. Results
1. Micro-Level
At the micro-level computerised medical records can be used to reliably identify people with CKD; though differences in creatinine assays, fluctuation in renal function, and errors in diabetes coding were less well understood. We also found significant errors in the coding of people with diabetes and end-digit preference in BP recording making this a blunt instrument to measure quality.
We became involved with CKD in collaboration with renal specialists interested in identifying people with CKD from general practice computer records [23]. Kidney function can be estimated using a simple formula to calculate eGFR. This is called the simplified Modification of Diet in Renal Disease (MDRD), which requires less information than other methods of calculating GFR; needing only serum creatinine (SCr), sex, age, and whether ethnic group is black, strictly Afro-Caribbean as this ethnic group has greater muscle mass [26] (Figure 1). As nearly all English general practices have a registered population (so age and gender are known) and lab-links meant that all SCr measures were readily available. Ethnicity recording was less complete [27].
There was some scepticism that computer searching was valid, we therefore hand searched 500 records to demonstrate that electronic searches were valid [28]. Once done routine data could be used to define the UK prevalence of CKD [24].
However, we later came to question these findings and revised downward our estimate of the prevalence of CKD as we learned more about inconsistency of creatinine assays and the fluctuation in individual patient's creatinine levels. The reliability of SCr assay and hence the diagnosis of CKD improved after 2006 when a national quality control system was put in place for creatinine assays [29]; however, prior to this it was necessary to adjust results to the assay used in the local lab.
Two important features emerged about fluctuation. Firstly, as creatinine fluctuates it is vital to have two readings at least three months apart, failure to use two readings results in an inflated estimate of prevalence by about 20% [4]. Secondly, we started looking at the degree of fluctuation in individual patients and found considerable variation [30]. We observed how there was considerable variation and sometimes improvement as well as decline in renal function. Figure 2 shows the variation in a group of females with diabetes; whilst the overall trend is one of reducing renal function with age there is enormous variation in individuals. The plot includes an attempt to plot a regression line above and below the fluctuating eGFR for each individual.
At the micro-level we also discovered issues with the diagnosis of diabetes [31]. We found problems with: 1) misclassification, most commonly people with type 2 diabetes who were incorrectly labelled as having type 1 diabetes; 2) miscoding, where people were given vague codes that did not differentiate the type of diabetes they had; and misdiagnosis, where patients were labelled as having diabetes but were not any treatment and did not blood test results compatible with the diagnosis.
Subsequent exploration of the medical records suggested that around 40% of the errors detected on the computer were of clinical significance [32]. Patients who were left off disease registers received suboptimal care [33].
Finally, we noted that there was marked end-digit preference. This is the preference for rounding BP and this data issue makes BP measurement a blunt instrument. There was also some suggestion of target bias, with a greater tendency to record a BP level just below the treatment goal level [34].
2. Meso-Level
At the meso-level we harnessed routine data to improve quality. CKD was a new concept in primary care and methods for estimating kidney function (eGFR) were not readily available. We filled this gap by developing calculators for phones and personal digital assistants as well as spread-sheets containing macros to calculate eGFR for a whole practice [35].
People in primary care were sceptical about CKD [36], lacked confidence, were inconsistent in their testing of kidney function [37], and the lack of confidence was associated with lower levels of achievement [38]. An educational intervention improved quality, again this improvement was measured using routine data [39].
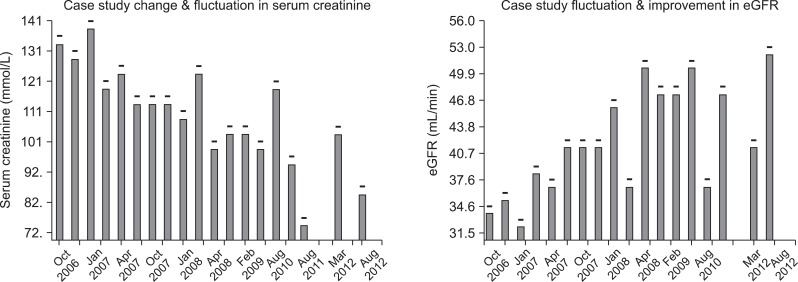
A case study of a single patient illustrates how IT helps identify and flag people with CKD who pre-2006 would have gone unrecognised in primary care. This case study concerns a widow, aged 79 years in 2006. Prior to the introduction of the CKD P4P target her kidney function would not have been considered abnormal for age (SCr, 132 mmol/L), and BP had been managed between a systolic of 145 and 185 mmHg. However, after the introduction of the CKD P4P her eGFR was calculated (34 mL/min) and her record automatically flagged as CKD. This led to more aggressive management of her BP. Her SCr has fallen and her renal function improved (Figure 3). This would not have happened without the automated flagging of this case.
3. Macro-Level: National Guidance, Pay-for-Performance
At the macro-level we helped ensure that leverage from informatics is incorporated in policy, and ecological investigations inform if there is any association with improved health outcomes. CKD management was part of a National Service Framework [40] with detailed guidance subsequently issued by the NICE [41]. NHS Employers, the contracting organisation, along with the British Medical Association commissioned knowledge support in the form of a set of Frequently Asked Questions monograph; this monograph was considered helpful by practitioners and is now in its third edition [42].
P4P was first introduced in April 2004, mainly targeted on vascular disease, with CKD domain added in 2006. This scheme uses routine data to determine the level of case ascertainment, on a disease register, and sets financially incentivised quality indicators. The CKD indicator includes a treatment target of keeping BP below 140/85 mmHg preferentially using angiotensin modulating drugs in the presence of proteinuria. Initial scepticism about CKD was replaced by improved primary care engagement in CKD management [43]. The author chaired the group that developed the CKD indicator.
An ecological study suggested that prevalence of diabetesand the proportion of people not at BP target, as recorded in P4P targets, could be added to the known predictors of variation in the requirement for renal replacement [25].
IV. Discussion
Informatics has been an important mechanism for implementing national evidence-based policy for CKD. It is hard to see how the rapid implementation of CKD guidance would have taken place so quickly without the IT infrastructure and processes in place and informaticians in support. Laboratories estimate renal function and these data are combined with data held on primary care computer systems to identify CKD cases that are then flagged for review and recall. This process runs smoothly, though there were teething problems that highlighted lack of standardisation.
At the individual practitioner and practice level, CKD was largely unrecognised prior to 2006 in English primary care. It subsequently started to be recognised and accepted and within a few years became part of mainstream practice. Policy was informed by the potential of EPR systems to identify cases of CKD and these systems were also used implement and monitor P4P CKD indicators.
The implications of these finding are that a combination of: technology, evidence-based guidance and health service management can achieve quality improvement. The context has been NHS policy to implement explicit evidence-based guidance, and to use P4P to incentivise that process. The informatics infrastructure and informaticians have been the mechanism to effect and measure change. However, in reality they have been intertwined rather than separate. The capability of the information system has informed policy, with a clinical informatician (the author) leading the development of the primary care quality P4P indicator for CKD. Notwithstanding these interdependencies standardisation of infrastructure, messaging and supporting informatics have been pivotal in this area of quality improvement [10].
The introduction of new technologies creates challenges; forcing the "actors" in a workplace to rethink what they do; in this case recognition and management of a new condition. From a socio-technical perspective this is a process of mutual transformation of organisation, clinical workflow and technology to manage CKD [44].
There are limitations to this study of the national implementation of improved CKD management. We have not proved a causal relationship between informatics and generalisability of these findings. A realist review provided a plausible mechanism for the role of informatics but others may suggest other mechanism being more important. Similarly a review of this type does not demonstrate generalisability, in that CKD is a relatively unique condition (like diabetes) which can be diagnosed entirely from numeric data contained within computer systems.
In conclusion, the right policy context informatics appears to be an enabler of rapid quality improvement. Informatics is not a magic bullet and we cannot prove a causal link. Informaticians working with clinical leaders and managers have contributed to the rapid implementation of CKD management into the English NHS.
Acknowledgments
Patients and practices who have contributed data and time to the research underpinning this journey, research colleagues, many funders including the Health Foundation who are the principal funders of the QICKD trial.
Notes
No potential conflict of interest relevant to this article was reported.