Wearable Sensors in Healthcare and Sensor-Enhanced Health Information Systems: All Our Tomorrows?
Article information
Abstract
Wearable sensor systems which allow for remote or self-monitoring of health-related parameters are regarded as one means to alleviate the consequences of demographic change. This paper aims to summarize current research in wearable sensors as well as in sensor-enhanced health information systems. Wearable sensor technologies are already advanced in terms of their technical capabilities and are frequently used for cardio-vascular monitoring. Epidemiologic predictions suggest that neuropsychiatric diseases will have a growing impact on our health systems and thus should be addressed more intensively. Two current project examples demonstrate the benefit of wearable sensor technologies: long-term, objective measurement under daily-life, unsupervised conditions. Finally, up-to-date approaches for the implementation of sensor-enhanced health information systems are outlined. Wearable sensors are an integral part of future pervasive, ubiquitous and person-centered health care delivery. Future challenges include their integration into sensor-enhanced health information systems and sound evaluation studies involving measures of workload reduction and costs.
I. Introduction
With respect to the consequences of the demographic change that most societies already experience or will have to face in the near future, challenges for the maintenance of the quality of care in health systems arise. Population estimates predict significant increases in the absolute and relative numbers of persons aged 80 years and above [1]. Changes will not only lead to a rise in the elderly part of the population and thus to a further rise in multi-morbidity [2], but also to a diminished workforce of caregivers as opposed to the number of persons in need of care. The latter trend is clearly observed when regarding the potential support ratio (PSR) of the United Nations [1], i.e., the number of persons aged 15 to 64 years divided by the number of persons aged 65 years and above. A marked decrease from 11.7 in 1950 to an estimated 2.7 in 2050 for the worldwide population can be observed. Europe's PSR will fall to 2.1 in 2050, China's to 2.4.
Information and communication technologies in general and 'health-enabling technologies' in particular are regarded as one among several means to support the maintenance of a high level of quality in care. Micro-electro-mechanical systems (MEMS) and especially sensor technologies may help to assess relevant signals and parameters that support caregivers and physicians in their work. While an increase of persons suffering from one or more functional impairments is expected, these persons often have a high motivation to stay in their familiar home environment and not in an institutional care facility. Sensor systems may support home-based or mobile assessment of a person's state of health and the data generated may be used to identify detrimental constellations or emergency situations. Thus, timely intervention and prevention measures may be taken to avoid further deterioration. This person-centered, ubiquitous care scenario demands new forms of living and care [3,4], and along with this a new kind of information system architecture with regard to health information. This architecture must include not only the personal or home environment as a source of relevant health data, but also the caregivers and other health professionals as opposed to current institution-centric architectures [5,6]. Such systems are called 'sensor-enhanced health information systems (seHIS)' [7]. Apart from their mere expansiveness, such systems must be able to provide context-dependent meaningful interpretations of the data gathered, in other words individualized decision support [6].
With regard to the rising relevance of wearable sensor technologies for healthcare purposes and the need for sensor-enhanced health information systems, the aims of this paper are
1) to summarize the state-of-the-art in wearable sensors for healthcare applications and to demonstrate their usefulness by presenting two current research examples, and
2) to review sensor-enhanced health-information systems with a focus on personal decision support systems.
The remainder of this paper is organized as follows: in the next chapter current wearable sensor technologies are presented, followed by a survey of application areas they are used in along with two examples from current research projects. The following section shows current approaches to seHIS with special regard on personalized decision support using wearable sensor data. The results are discussed critically and future research demands are identified.
II. Wearable Sensors
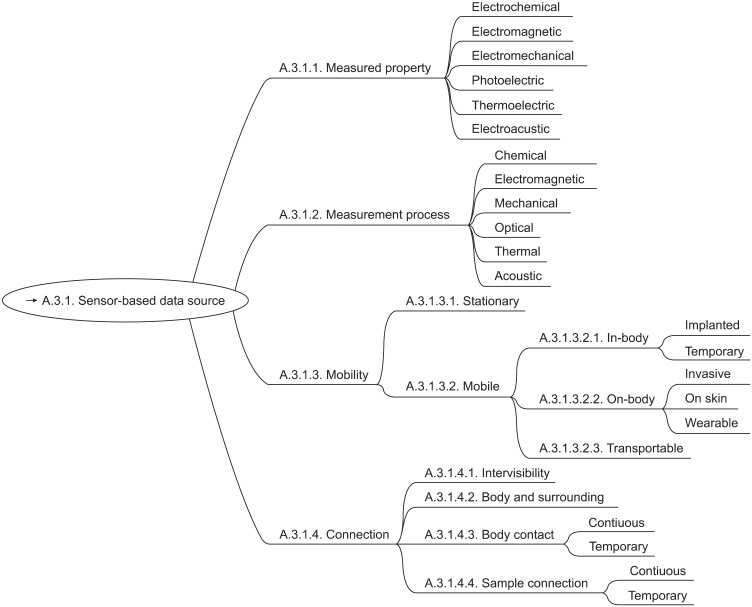
The ideal sensor-system for health related parameters would be deployed at one point in time and continuously measure and wirelessly report all health-related information thereafter. It would not constrain or affect its user in any way and it would need no maintenance. Until now systems like the outlined one are still science fiction, but taking into account the technological advances of the last decades it seems obvious that in the not too distant future systems like this will be feasible. Nowadays existing systems still struggle with the above-mentioned requirements. One of the biggest problems is energy consumption, implying the need to recharge and service the devices frequently. This in turn influences the acceptance and compliance. The gap between the amounts of energy that is harvestable and the need of current sensor systems is still large, but shrinking. The need to service the devices is not only due to energy management, but depends on the measurement process and the connection between sensor and sensed subject as well. In [8,9] we proposed the four axes mobility, connection, measured property and measurement process to organize sensors for health related parameters and later improved the scheme (Figure. 1, [10]). In the recent development of the Systemized Nomenclature of Contexts, Analysis and Problems in Health-Enabling Technologies (SNOCAP-HET) the proposed systemized taxonomy for sensors was utilized as part of the context axis [10].
The existence and availability of wearable sensor systems for health-related parameters that are easy to deploy and no burden for the patient is still one, if not the major factor hampering the adoption and establishment of health-enabling technologies. The currently deployed systems have to balance the trade-offs in the different categories.
III. Sensor Application Areas in Healthcare
1. Future Needs of Care and Application Scenarios
Large-scale cohort studies such as the Berlin Aging Study [2] have shown that aging is related to increased prevalence rates of chronic diseases and thus to a rise in multi-morbidity. Steinhagen-Thiessen and Borchelt [2] report a prevalence rate of 88% for five or more somatic diseases in the population aged 70 years and above. While it is expected that the 'future elderly' will be healthier than persons of the same age in the past and therefore less likely to be in need of care, sheer numbers associated with demographic change will outweigh this effect. Thus a closer look to some epidemiologic predictions is worthwhile.
Comprehensive data concerning the mortality attributed to different disease conditions have been published from the WHO's Global Burden of Disease study by Mathers and Loncar [11]. These data predict (for the year 2030) the highest world-wide mortality to be caused by ischaemic heart disease (13.4%), followed by cerebrovascular disease (10.6%), HIV/AIDS (8.9%), chronic obstructive pulmonary disease (7.8%), and lower respiratory infections (3.5%). The first two conditions share underlying risk factors and have a common pathology, rendering cardiovascular diseases as the most important ones with regard to mortality. Looking into prevalence data, however, we find other conditions that cause disability and reduction in the quality of life, yet do not necessarily lead to death. Goldman et al. [12] have published prevalence predictions based on Medicare data for the year 2030, showing that arthritis (68.4%) and hypertension (58.8%) will affect more than half of the population aged 65 years and above, while diabetes mellitus is attributed to the highest average costs per individual among the five leading conditions.
In order to assess the individual and societal impact of different disease conditions, the WHO has introduced the 'disabilty-adjusted life years (DALY),' a metric defined as the 'sum of life years lost because of premature death and the years lost to disability' [13]. Based on the baseline predictions of WHO data as published by Mathers and Loncar [11] for persons aged 70 years and above in high-income countries, it can be observed that cardiovascular diseases have by far the highest impact, followed by neuropsychiatric and malignant diseases (details of these calculations can be found in [14]).
Cardiovascular diseases have long since been in the focus of wearable sensor technologies, also because wearable systems to record electric signals have been available since the 1960s (Holter [15] monitor) and have seen steady miniaturization and refinement. The second-most important group of conditions, however, is the group of neuropsychiatric diseases (e.g., dementias, uni- and bipolar depressive disorders) whose impact is rising. Only comparably few technology projects address conditions such as uni- or bipolar diseases, while a recent report of the World Economic Forum and Harvard School of Public Health has shown that 'mental illness' is expected to cause even higher costs (16.3 trillion US$) than cardiovascular diseases (15.6 trillion US$) among all non-communicable diseases within the next 20 years [16]. We may conclude that apart from the well-known application field of cardiovascular diseases, neuropsychiatric conditions gain more and more importance and therefore should be addressed even more intensively in future research projects.
2. Research Examples of Wearable Sensor Applications
The following two examples-fall risk assessment and biomechanical joint function analysis-were chosen in order to demonstrate the use of wearable sensor systems for healthcare applications. However, the authors wish to emphasize that there are numerous advanced other projects using wearable sensors which are not referenced here explicitly.
3. Fall Risk Assessment in Dementia Patients
The number of patients suffering from dementia is expected to double every 20 years in the context of the demographic change [17]. The worldwide costs for dementia in 2009 were estimated at 422 billion US$ [18]. A large amount of these costs is caused by the high risk of falling associated with dementia [19,20].
Fall prediction is an ongoing field of research and a lot approaches have been developed to identify fallers in hospitals [21]. These approaches can roughly be classified in fall risk scores and geriatric assessment tests. Assessment tests aim for measuring the self-contained mobility. Typical examples for assessment tests are the Tinetti [22] test and the timed 'up & go' test [23]. Research has shown that it is possible to draw conclusions about fall risk from these measurements, because self-contained mobility and especially gait are one of the key issues of fall risk.
In order to increase the objectiveness of assessment tests, recent studies aim to employ wearable sensors to enhance their predictive ability. Especially accelerometers have been used to measure movements performed in these tests [24-27]. The advantages of accelerometers are small size, increasing accuracy, low power consumption and acceptance by patients.
Physicians and physiotherapists which are supervising assessment tests know that the patient will change his or her behavior when he or she is being studied. This so-called Hawthorne effect can be minimized if the patient is not supervised by another person, which can be achieved by wearing the sensor during the patient's everyday life. After a while the patient will ignore the sensor. Another advantage of unsupervised settings is the continuous investigation. Thus, the subjects may be measured over extended periods of time and not only during a specific test with none or just a few repetitions. This setting will strongly increase the amount of available data and holds logistical as well as algorithmic challenges. Gait periods have to be detected automatically. This can be done using auto-correlation methods [28]. Due to the fact that it cannot be expected that the sensor is always aligned in a certain way, virtual alignment to the axes of the human body has to be done [29]. Afterwards, gait parameters such as velocity, kinetic energy or compensation movements can be computed from each gait period. The computed gait parameters can be processed and analyzed with respect to fall events recorded in institutional fall protocols using data mining techniques, with the aim to identify persons at risk. Fall risk assessment and fall prevention may be efficiently implemented in nursing or retirement homes.
4. Biomechanic Studies under Daily Life Conditions
Arthritis of the knee (gonarthritis) is one of the most common forms of arthritis in the elderly and frequently causes lasting functional limitations. End-stage gonarhritis is often treated surgically with a knee endoprosthesis. As this disease not only affects elderly but also an increasing number of younger persons, the expectations are high with regard to functional outcome after surgery and especially the ability to perform sports activities. Recently, new techniques and implants have been developed allowing for replacement of only the affected parts of the knee joint instead of conventional total replacement. This technique is less invasive and bone-conserving and does not remove the cruciate ligaments which are important for stability. Studies have shown that this may have beneficial effects like a better functional outcome for kneeling or walking downstairs [30], and for reaching pre-operative activity levels more quickly [31] compared to the conventional total knee replacement. At present, however, functional assessment is often confined to gait lab environments and everyday-life-activities cannot be observed by the clinician or physiotherapist. The outcome of different surgical techniques with regard to such activities in an unsupervised environment can be evaluated by using wearable sensor devices as a supplement to the conventional subjective self-rating scales (such as knee scores [32]).
Recent research has shown that approaches combining accelerometers, gyroscopes and magnetometers provide the ability to capture pelvis and knee joint kinematics [33-36]. Practicable measurements suitable for a patient's daily use face specific challenges. Using the KINEMATIC WEAR system developed by our research group [37], sensors are attached to the patient's skin at appropriate positions on the pelvis, thigh and shank with a flexible tape to minimize motion artifacts. The sensors should neither support nor constrain knee or pelvis motions to avoid distorted results. After calibration and virtual alignment of the sensors to the bone axes, time-synchronization of the three sensor nodes is performed. Noise, transients and drift of the sensor data can be filtered out with a band pass filter and a linear model.
In a prospective study setting, a validation trial of KINEMATICWEAR against a reference marker-based video gait analysis system (VICON Motion Systems Ltd., Oxford, UK) was performed, indicating a sound quality as well as a high degree of correspondence [38] (correlation of 0.98-0.99 for sagittal knee angle measurements). Applied now for the purpose of supporting clinical evaluation before and after surgery, entirely satisfying results can be reached by measuring gait activities during everyday life outside the lab. Especially during challenging activities where stability of the knee after a total knee replacement is supposed to be limited, markers such as changes over time in activity levels, of compensation movements or gait symmetry can be identified thanks to continuous ambulatory monitoring with unobtrusive mobile body sensors.
IV. Sensor-Enhanced Health Information Systems and Personalized Decision Support
With the advent of sensors for health related parameters that are good enough to base medical decisions on and cheap enough to be deployed in larger cohorts, the management of the data gathered becomes an issue. Sources will not only be sensor systems produced by medical device manufactures and prescribed by physicians [39], but sensors in devices of everyday life bought and used on an individual basis (such as smart phones, cars, smart homes, etc.) [6]. While continuous and ubiquitous measurement of parameters potentially produces new and valuable imagery of diseases' onset and, subsequently, therapeutic effectiveness on an individual, personalized basis, the analysis of the huge amounts of heterogeneous data must be performed automatically. Due to the inherent plurality of data sources, it is unlikely that classical approaches developed for the management of information systems of one or a few often similar facilities will be sufficient for the emerging seHIS. The same applies for the technical part of the information system, as many data might be captured for non-health related uses and medical exploitation may often be a by-product.
A literature review of current sensor-enhanced information systems has revealed that most approaches address specific medical conditions or problems and that, while necessary parts of the information system infrastructure are implemented, others are left out [7]. Among the many requirements for successful implementations of seHIS, the following aspects may be regarded as crucial [7]:
Person-centeredness: Instead of institution-centered architectures, seHIS should support person-centered care, involving many data sources [9,40]. This shift away from institutional towards person-centered records implicates the implementation of strict data security rules and measures, e.g., the usage of authentication methods as presented e.g., by Gomez et al. [41].
Standardization: Not only the device interfaces should be standardized [42], but also the representation of data and information [43,44] and of decision logic [45,46].
Multi-modal mass data analysis: Integrating multiple heterogeneous data from different sources (and of different quality) requires adequate data fusion, reduction and analysis techniques in order to extract relevant information from the data [8,40,47,48].
The challenges, however, are not only of technical nature [49], and health care will gradually incorporate wearable sensor technologies.
Personalized decision support incorporating a person's sensor data is frequently named as a crucial component for personalized health care in [50-52]. Intensive research work is conducted in the field of personal decision support components, ranging from single-parameter analysis systems [53] to advanced multi-modal decision support infrastructures [47]. The requirements of such systems, apart from their capability of being integrated into a seHIS infrastructure (interfaces, data security, etc.), include real-time response, alert prioritization, customization of the decision logic and provision of comprehensive explanations of decisions taken. A detailed review can be found in [53].
IV. Discussion
In 2008, Saranummi and Wactlar [54] have stated that pervasive healthcare as a field of research '... is still a nascent one, with a good deal of exploratory research.' Wearable sensors are without doubt an integral part of the pervasive healthcare vision, and sensor technologies are, despite several issues such as power consumption respondent battery lifetime which are still to be resolved, already advanced with regard to their technical capabilities. While many projects have demonstrated the benefits of using sensor-based monitoring for specific medical problems such as cardiac arrhythmias, diabetes or heart failure, wearable sensors represent just one piece of the puzzle, because sensor data have to be interpreted individually in the context of all available health information, e.g., existing in institutional electronic health records. Therefore, the integration of sensor-based systems with clinical/institutional and other health professionals' information system components into a trans-institutional, sensor-enhanced health information system is a necessity if complex decision making shall be facilitated. Individualized decision support has frequently been pointed out as a prerequisite for personal health systems [5,52], and it still is a major challenge because of the numerous issues that have to be resolved when implementing seHIS structures such as common terminologies, semantic interoperability or the standardization of device interfaces, representation formats for clinical and sensor data and decision logic [7].
Thus, from the authors' point of view, wearable sensor technologies in the context of healthcare should not be regarded as a secluded field of research but as part of an interdisciplinary research effort in which sensor data provide relevant additional information to be used for individualized decision support within a seHIS. This view among other things implies that there is a need for sound validation studies in close collaboration with nurses, physicians, physiotherapists and other health professionals proving that wearable monitoring really does provide additional information and thus does have therapeutic consequences. With regard to the motivation to use wearable monitoring to mitigate the consequences of demographic change in terms of decreasing caregivers' workload and of reducing healthcare costs, there is still a need for such evidence. It is obvious that continuous monitoring of numerous health-related parameters is neither efficient nor desirable for everyone and that deployment should be well-considered, evidence-based and individual.
Will wearable sensors in healthcare and seHIS be 'all our tomorrows' and more than a 'brief candle'? Yes and no. Continuous and frequent health monitoring under unsupervised, daily-life conditions as shown in the two examples above can provide additional information relevant for diagnosis, therapy or prevention and may even support patient empowerment and independence. Yet it cannot resolve all problems and should be applied in due consideration of the advantages and drawbacks of this technology.
The International Medical Informatics Association (IMIA)'s working group on 'wearable sensors in healthcare (www.wearable-sensors.org)' addresses research issues related to the above-mentioned challenges and the first author cordially invites all persons interested to participate in the group's efforts.
VI. Conclusion
This paper summarizes current research in wearable sensors for healthcare and sensor-enhanced health information systems. Intensive research efforts are undertaken in this field which evolves rapidly, yet there still is a need for large-scale evaluation studies apart from several technical and organizational obstacles to be overcome.
Notes
No potential conflict of interest relevant to this article was reported.