Electro-Mechanical Safety Testing of Portable ECG Devices for Home Healthcare Usage
Article information
Abstract
Objectives
This paper suggests the experimental guidelines to evaluate the electro-mechanical safety of belt type equipment. The electro-mechanical safety was determined by using the International Electrotechnical Commission guidelines, which are widely used as important factors for assessing the electro-mechanical safety of belt type equipment. However, the local guidelines on wearable healthcare sensors are currently not well-established. Therefore, safety guidelines suited for the actual circumstances in Korea are required, and this paper attempts to try a new experimental safety test procedure of the wearable healthcare sensor.
Methods
This belt type device measures the electrocardiogram (ECG) and heart rates by attaching to the chest. Examination lists were selected by analyzing the common standards ofelectro-mechanical safety (IEC 60601-1) and environment tests (IEC 60068-1, IEC 60068-2) of home-healthcare equipment.
Results
The essential electrical safety, which was required for the RS300G3 as a medical device, was evaluated, and most of the examination lists were selected by considering the circumstances of the users. The device passed all the selected examinable lists that are applicable to the Korean environment.
Conclusions
This study has limitations to estimate and to conduct electro-mechanical safety experiments because our study focused on the belt type of heart-rates equipment. We are not taking into account the overall electro-mechanical home-healthcare measurements. According to industrial and technological development, there are infinite possibilities for the advancement of home-healthcare equipment, so more examination lists for safety are being added in addition to what we have done.
I. Introduction
Nowadays, in the medical field, ubiquitous home-healthcare systems are added to medical services, and home-healthcare system development researches are actively progressed [1]. Home-healthcare equipment developments which make it possible to check one's heart, blood pressure, and change of blood glucose without visiting the hospital are matters of concern [2,3]. Therefore, equipment for health diagnosis and management is becoming practicable. Developed equipment enables the medical home-healthcare system linked to the network to support medical healthcare services such as prevention of diseases, diagnosis, and management.
Home-healthcare products are used by untrained people at home without the supervision of public health experts. This results in many possible dangers including poor power supply or electrical arrangement in the environment. Therefore, the electro-mechanical safety is demanded for the properties of users or surroundings. In addition, to ensure the correct use and safety of home-healthcare equipment connected with the network, the reliance of electro-mechanical safety and circumstances on all occasions should be guaranteed. The standard for electro-technical safety measurements and methods is required to prevent patients from unexpected medical accidents like errors or faults in home-healthcare equipment, and careless use. To present the standard for electro-technical safety measurements and methods of home-healthcare equipment, it is necessary to follow the existing International Electro-technical Commission (IEC) standards. The standard for requirements and efficiency of home-healthcare devices also needs to be examined, because this study focuses on wearable healthcare sensor equipment. Home-healthcare equipment is defined as a medical device.
Since electrical equipment could be guaranteed for electro-mechanical safety and efficiency, we are studying the special qualities of the wearable healthcare sensor equipment. An object of this study is to evaluate how the devices are safe to people using the wearable healthcare sensor equipment without training and to suggest the experimental guideline which is adjusted in Korean circumstances. In this study, B (referred to as B in Figure 1) is applied to the belt-type wearable healthcare sensor equipment.
II. Methods
1. A Wearable Healthcare Sensor Equipment
A measuring instrument including a belt and a watch (made in the A Corporation) was used for detecting heart rates. This belt-type equipment measured heart rates, physical strength, and exhausted calories, and saved the personal data in the watch simultaneously. The watch transmitted the data to the personal computer by using an infrared light transmission.
Personal data from the watch was analyzed in the computer to display the exercising time and distance. Weak heart releases the blood quickly because the body needs abundant blood, and then heart rates are increasing [4].
Therefore, if people check their heart rates, they can confirm their heart health condition and the circulation of blood. People check their health condition through the a trainer software provided by a corporation. In this study, the watch in Figure 1 was selected for the electro-mechanical safety and efficiency measurements.
2. Classification
To evaluate the safety measurement of the belt (referred to as B in Figure 1) as wearable healthcare sensor equipment, the standards of electro-mechanical safety should satisfy by the IEC 60601-1, general requirements for basic safety and essential performance. According to this standard, medical equipment is classified into two types: external and internal power supply types, followed by protection from the electrical impact.
General home-healthcare equipment is offered with batteries or chargers connected with an external power supply, and this equipment used in this experiment corresponds to the internal power supply type. B also corresponds to the internal power supply type using batteries. Therefore, B should satisfy the experiment lists which are essential to the internal power supply type. Moreover home-healthcare equipment is classified according to use circumstance: hand-held or portable types. B is a suitable device for the portable type, so B should be applied to experiment lists as the portable type.
3. Electro-Mechanical Protocols
The B evaluates the leakage current, dielectric voltage, internal impact, overheating, surfaces, edges, and boundaries experiment for electro-mechanical safety measurements.
The leakage current signifies that current leaks irrespective of the equipment' f unctions, and that current is classified into three types: earth, enclosure, and patient leakage current. As ground is not required for B, earth leakage current does not need to be measured. If the permissive voltage is increasing gradually, there is the time when the equipment is damaged. At this time, the current voltage is represented as the dielectric voltage. The isolation part of the B should withstand the reference voltage for one minute. B should be designed to protect from contacting with the other part which is possible to become a flowing current. The internal impact experiment implies inner damages when force is added from the surface to the inside of the equipment. External parts and components attached to B should not be damaged in whole parts of that surface (extending over 625 mm2) when 45 N power is added to inboard direction directly, and it should be no damaged when 0.5 ± 0.05 J shock is added by the shock tester. Rough surfaces, sharp edges and boundaries which can cause trauma or damage should be covered or removed.
4. Environmental Experiment Protocols
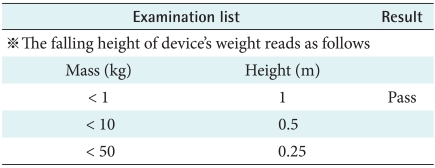
As home-healthcare equipment is not used in fixed place such as hospitals or clinics, it can be damaged by dropping, spilling, and dust. This study performed the experiment of mechanical strength of the wearable healthcare sensor equipment in diverse situations. According to IEC 60068-2-32, B's malfunction was measured by free fall from 1 m height. The B was classified as drip-proof equipment, and evaluated for damage when it infiltrated by water or particles.
III. Results
1. Electro-Mechanical Safety Measurement
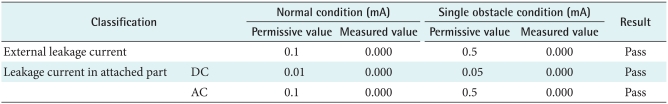
1) Leakage current and dielectric voltage
When a patient puts on healthcare device, the leakage current from the equipment can cause an electric shock. Therefore this study examined each permissive value and measured value in normal conditions and single obstacle conditions as shown in Table 1 for patient's safety. As a consequence, measured value like external leakage currents and dc/ac leakage currents in the attached part were not over the permissive values so leakage current and dielectric voltage factors passed the test (Tables 1, 2).
2) The internal impact experiment
External parts and components attached to the equipment should not be damaged in whole parts of that surface (extending over 625 mm2) when 45 N power is added to inboard direction directly, and continuity and space distance should not be reduced under the reference numerical value regulated by 57.10 in IEC 60601-1. Moreover, external parts and components attached to the equipment should be no damaged when 0.5 ± 0.05 J shock is added by the shock tester regulated by an appendix G in IEX 60601-1, and not be inconsistent about the requirements regulated by 3.44 and 57.10 in IEC 60601-1. As a result, the first reference had no damages and the second had no safety so these examination test are passed.
3) The exterior and protection covers of RS800G3
Fastening specifications are separated from live electricity and also fastening specifications is separated by dual insulation or strengthening isolation (Table 3). As a result, no apertures were protected by the exterior covers, so the equipment passed the test.
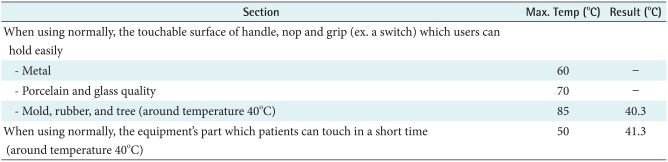
4) Overheating
The B was made with rubber so it would be affected by heat. Therefore, this study evaluated heating tests based on IEC common standards. According to IEC, rubber's maximum temperature is 85℃ but our result is 40.3℃. When it used normally, patients can touch in a short time. In this case the result is 41.3℃. Therefore, the temperature is starting from 40℃ to 41.3℃. Eventually increasing value is 1.3℃, so the device wasn't overheating (Table 4).
2. Environmental Tests
1) Degree of protection provided by enclosures
According to IEC 60529, the device and its exterior were classed as drip-proof equipment. An access probe (Figure 2) which a diameter of 2.5 mm did not passed. As a result, the water does not affect device when sprayed 60 degrees on both sides on a vertical plane (Table 5).
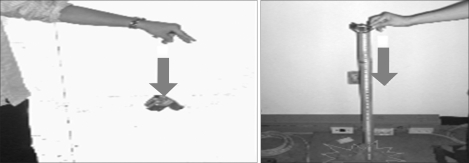
2) Motion of free fall
The portable device should be stand stress under the rough situation. The device would have not disorder on these conditions that the products is holding up to height (Reference IEC 60601-1's chart VII) and let fall from that height to the woodcut which is made by 1.5 times, a thickness of 50 mm and set on a hardness base (Figure 3). As result, when users drop the device, there is no problem to use equipment continuously (Table 6).
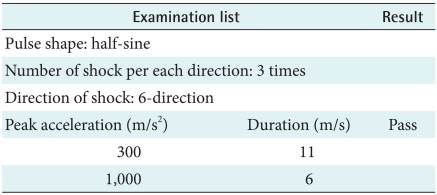
3) Shock test
According to IEC 60068-2-27 (1987), the device should not cause the malfunction during the vibration test and after. Moreover, when a maker uses the device, it should perform regulated function within tolerance limits continuously. This test of aim is the case of fell down so the test's result show there's no problem and the device doesn't bring about dangerous factor (Table 7).
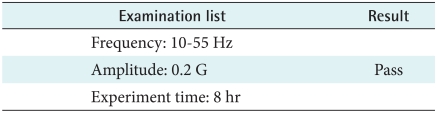
4) Sine wave random test
In accordance with KS C 0240 (1998), the device should run for 8 hours normally after testing which was set frequency, the amplitude and experiment time. Therefore when users drive in a car, the device has no problem (Table 8).
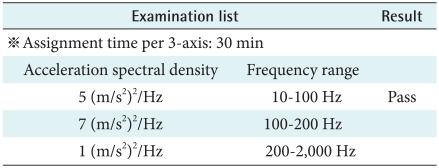
5) Random vibration test
According to IEC 60068-2-64, the equipment should not bring about dangerous factors when it use or later. Therefore the device continuously operates regulated functions under tolerance limits. Therefore when users ride a car, the device has no problem. In addition when users do exercise, the device has no problem (Table 9).
6) High temperature and high humidity test
According to KS C 0222 (1989), a constant temperature water tank was set to 40 ± 2℃, humidity 93+2-3 and maintained this setting during 96 hours. Next, the equipment put in the water tank and takes out. If was left on the desk at room temperature. The device had no problem, and users can put on equipment in hot weather and high humidity.
IV. Discussion
The electro-mechanical safety test of portable belt type ECG devices which was essential as medical device was evaluated. Home healthcare products are often used in environment without skilled supervisor and well-defined information with electronic facilities and safety. Moreover, home healthcare consumers are usually elderly so they are not good at using electro- mechanical devices and they do not know well about the danger of electricity. Therefore these tests are required for maintaining the safe way of using.
This study focused on the environmental safety of medical device than the device's own performance. Other essential safety efficiencies, battery condition indication, function of defect examination, visible and auditory alarm system like software category was not considered. At first, the B device which is used in this test measured leakage current, overheating, overheating and surfaces, edges and boundaries test for checking fundamental electro-mechanical safety. Then for an environmental point of view, this study examined free fall, watertight and protection against dust test, salt water spray test, high temperature and high humidity test.
Therefore minimum examination lists were selected and evaluated in considering circumstances of users. B was satisfied the experiment lists about electro-mechanical measurement's basic safety and essential performance for inner power supply. In the environment test, B's interior was not penetrated by water and particles. On free fall testing which was about dropping from one meter height to a rigid base and there was no problem for B's operation.
In conclusion, this study suggests that these electro-mechanical safety guidelines are suitable to determine the belt type ECG devices as wearable healthcare sensor measurements. According to industrial and technological development, there are infinite possibilities and variety for the advance of home-healthcare equipment, so more examination lists for safety are being added as well as what we have done.
Accordingly it is necessary for us to achieve more desired examination lists in all conditions; the electromagnetism harmfulness evaluation against the human body, the electro-magnetism suitability and the device's efficiency.
Acknowledgements
This study was supported by a grant (08142KFDA369) from Korea Food & Drug Administration (KFDA) in 2009 and the Ministry of Knowledge Economy (MKE) and Korea Industrial Technology Foundation (KOTEF) through the Human Resource Training Project for Strategic Technology and a grant of the Seoul R&BD Program, Korea (10526) and the Brain Korea21 Project for Medicine Science Yonsei University, Korea.
Notes
No potential conflict of interest relevant to this article was reported.